Improper disposal and DEA reporting of pharmaceutical materials can cost healthcare facilities thousands in regulatory fines.
When it comes to properly managing waste streams per regulatory requirements, healthcare facilities are constantly searching for solutions that not only protect them from regulatory infringements but can do so without being locked into long-term contracts from waste pickup services which threaten them with large financial penalties if they decide they want out early.
This problem is exasperated when facilities are trying to find proper solutions for the disposal of DEA Controlled Substances, which involves additional cradle-to-grave tracking documentation paired with DEA-certified reverse distribution services.

A new way to manage your pharmaceutical waste.
HealthFirst Environmental has partnered with Reliable Pharmaceutical (RP) Returns to provide your customer with a smart, compliant, and economical way to manage non-controlled and Schedule II-V controlled substances using DEA compliant mailback services – no contracts required!
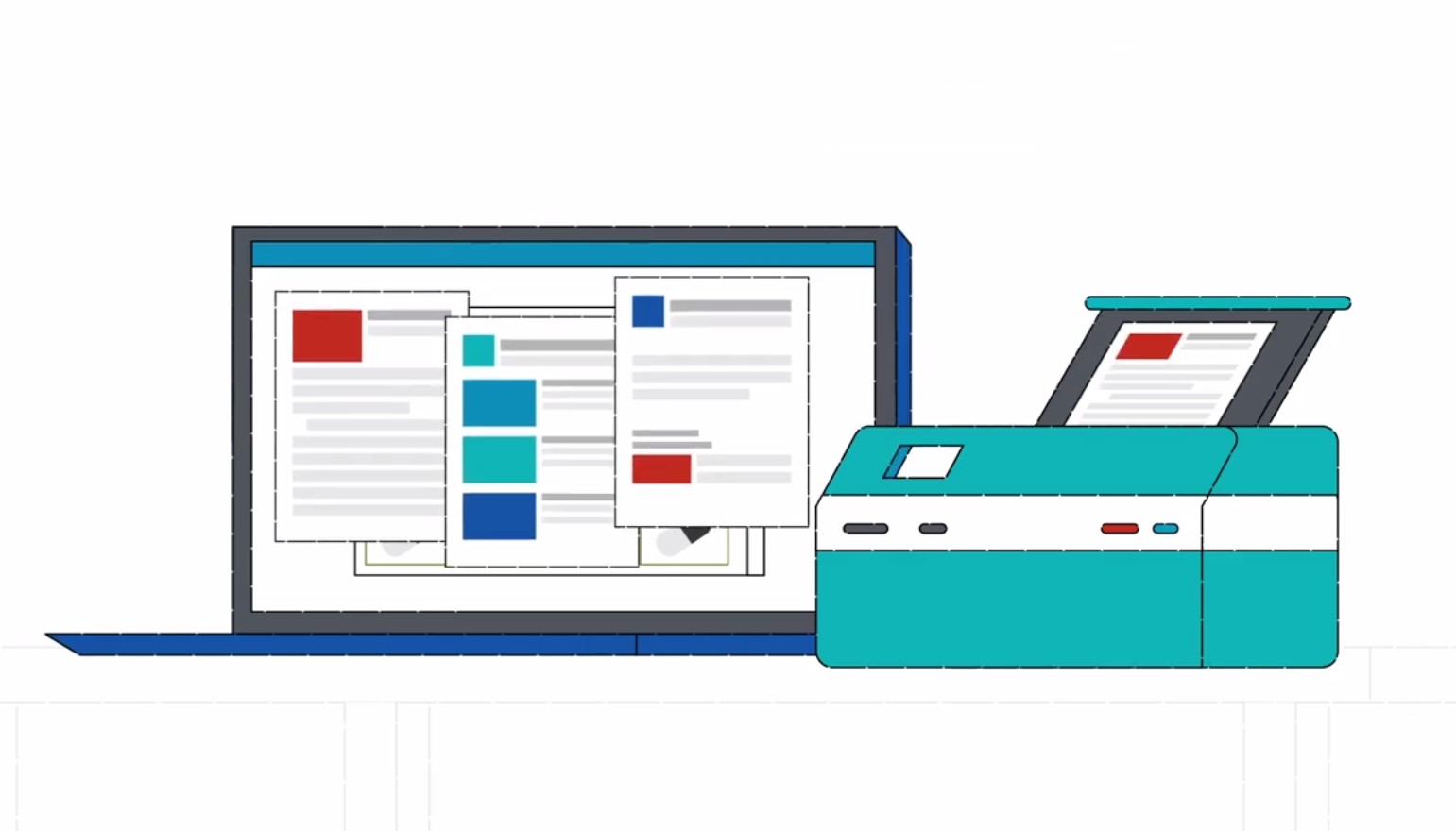
Online Client Returns Portal
This service includes online access to a proprietary Client Returns Portal interface, which allows the user to prepare and print DEA required return manifests prior to return shipment. All DEA paperwork and processing services is conveniently provided by RP Returns, a certified reverse distributor for non-controls and DEA Controlled Substances.

Pack, Ship, Report, Done!
- Place all pharmaceutical materials into the postage-paid return box along with the printed manifests.
- Give the completed box to a UPS driver or deliver it to your local UPS drop-off location.
- Once the box is received and processed by RP Returns, an email will be sent to the contact email address with a link to download the completed Processing Certificate.
- Keep a copy of the processing certificate for proof of processing. You are done!

